About Fuel Cells
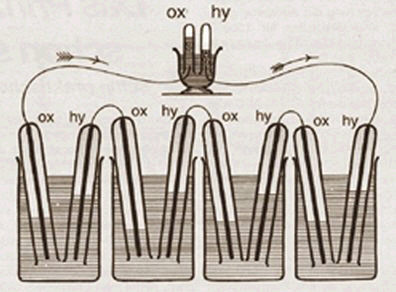
Fuel cells are devices that produce electrical power, using the chemical properties of a fuel (often hydrogen) and oxygen to directly create electrical current. They are technically similar to a battery, although, unlike a battery, they do not store energy, but produce electricity as you need it from an external fuel source
They were initially demonstrated in 1839, by Sir William Grove;however, a truly workable fuel cell was not demonstrated until 1959. After use in NASA's space programme, interest in fuel cells died down somewhat until the 1990s when they were considered as a replacement for combustion engines, because of their potential to be a more efficient and clean way to create power.
There are a number of types of fuel cell which are normally distinguished by the electrolyte they contain. The best-known types are alkaline, molten carbonate, phosphoric acid, proton exchange membrane (PEM) and solid oxide. Direct methanol and regenerative fuel cells are also being extensively researched.